Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's standard dummy text ever since the 1500s
Lorem IpsumIntegrated contract research, development and manufacturing organization (CRDMO) for better results, faster.
We provide end-to-end support across the entire drug development lifecycle from small molecule APIs, combining the deep science of a contract research organization (CRO) with the fast efficiency of a contract development and manufacturing organization (CDMO).

Our seasoned team of medicinal chemistry experts have decades of specialized experience in solving the most complex synthetic challenges. For more than 20 years, we have designed hundreds of compound analogs and facilitated the synthesis of thousands of variants in support of target identification through lead optimization, across therapeutic areas. Wilmington PharmaTech has participated in rescuing many promising molecules that other contract providers could not develop.
In addition to chemical synthesis solutions, through our partner network, we can also coordinate other services supporting drug metabolism and pharmacokinetics (DMPK), safety, toxicology, product development and clinical trial support.

We provide Better Science Faster! For more than two decades, Wilmington PharmaTech has invested in creating deep scientific experience in chemical development for IND-enabling, seamless support. This includes process development and process scale up, through to cGMP supply. Our facility capabilities are purposefully designed to handle the full range of drug substance challenges, including multi-chiral center, OEB 5 HPAPI (e.g., for cytotoxic compounds and antibody-drug conjugate (ADCs)), continuous-flow processing, controlled drug substance (CDS), and peptides. Our capabilities include:
- API synthesis
- HPAPI dedicated suites
- Process development and optimization
- Process scale up
- Analytical method development, validation and ICH stability programs
- API impurity services
- Robust IND-enabling services
We continuously invest to keep our laboratories and cGMP suites current, and well-equipped, including the recent addition of scalable purification solutions for chiral and achiral separations. Our advanced purification services include racemates and atropisomers with the same cycle used for the purification of both chiral and achiral molecules.
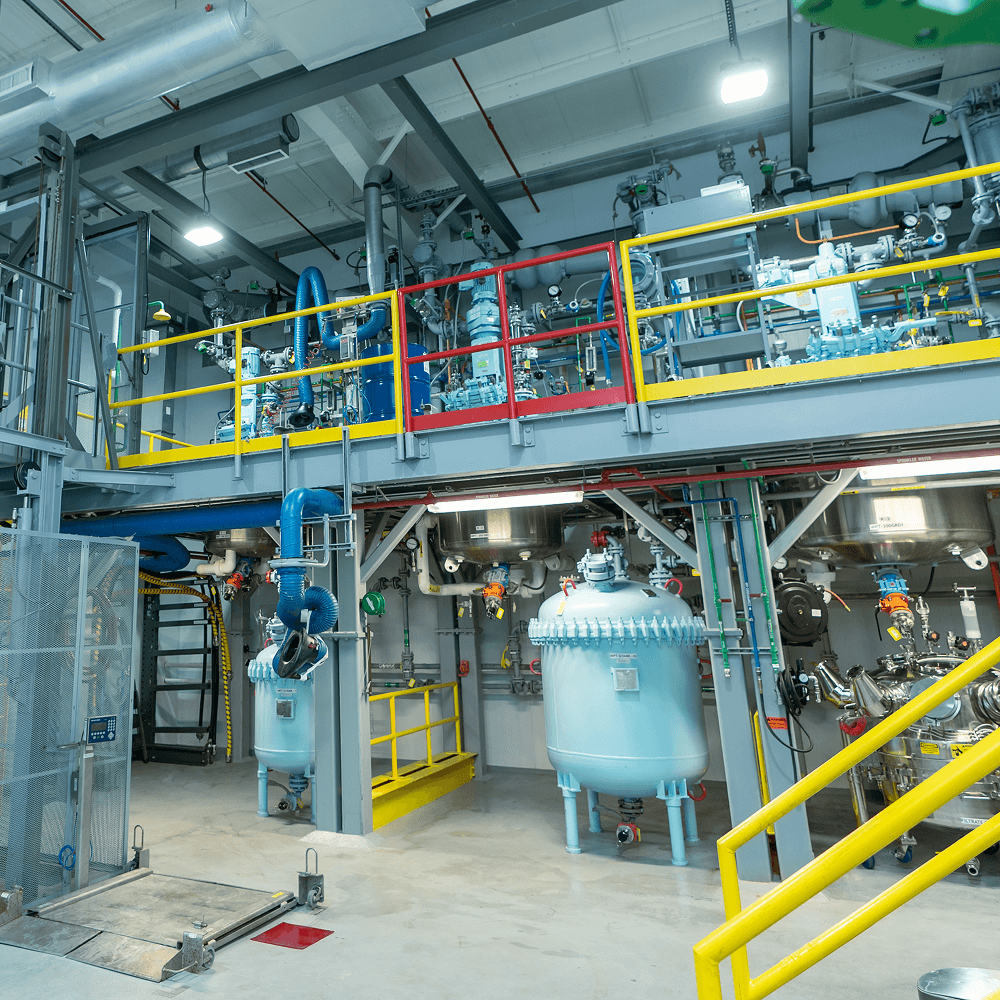
Proven expertise to accelerate your discovery program through target identification, scaffold synthesis, and lead optimization.
- IND-enabling to small-scale commercial pilot plant for API production
- High-potency API (HPAPI) handling
- Capacity from large-scale cGMP labs to pilot suites
We offer flexible sourcing and support options to accelerate milestones, scale up production from pre-clinical to commerical volumes, optimize manufacturing productivity, and drive cost-efficiency.

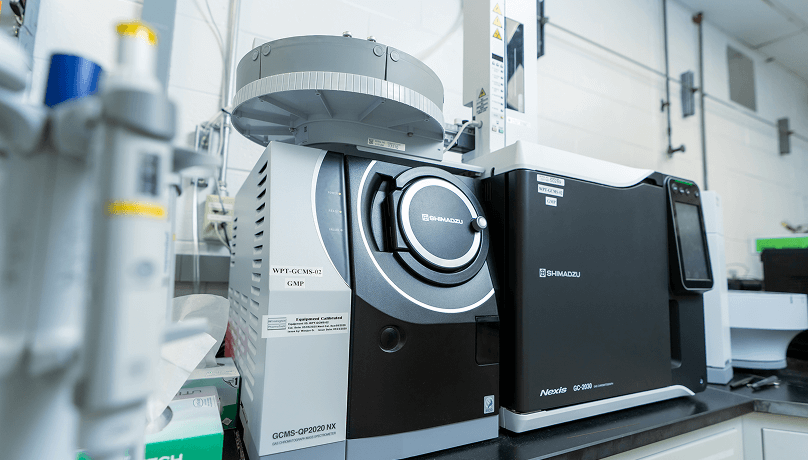
Whether for in-process, release, or stability testing, Wilmington PharmaTech provides a comprehensive suite of analytical testing capabilities, including:
- Analytical method development and validation
- Full cGMP release for both clinical and commercial API
- Non-GXP and GLP testing
- Reference Standards, including preparation, qualification, and distribution standards for intermediates and APIs
- Identification of impurities and preparation for qualification, retesting and distribution of impurity standards
- Full ICH stability studies, including trace impurity analysis
- Designated QA office and documentation storage
- Genotoxicity impurity services including: assessment reports; reference compounds; Ames test (via partner); control strategies; analytical method development, qualification and validation; sub-ppm trace impurity analysis for RSMs, APIs and intermediates; final technical reporting.
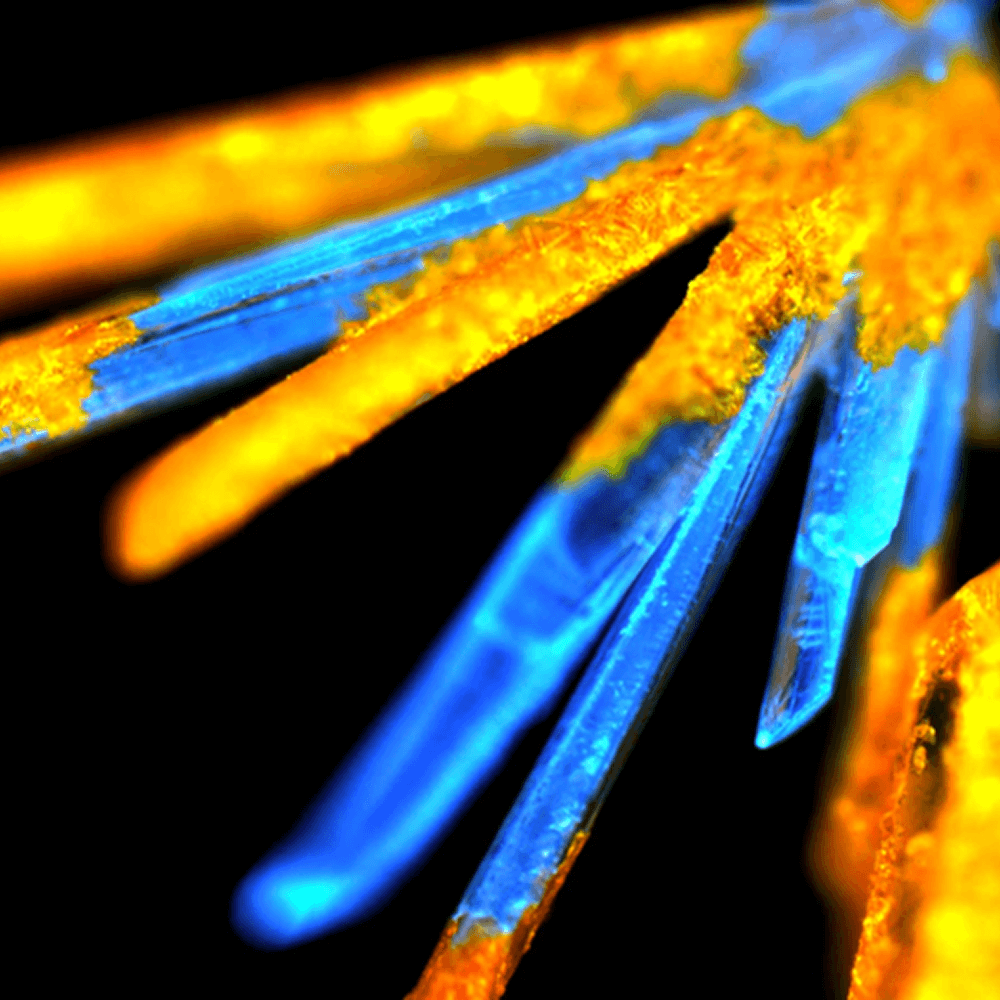
With a wealth of expertise and a combined 20+ years of experience, Wilmington PharmaTech provides comprehensive solid-state chemistry support to optimize your API’s form. Our services include:
- Salt selection and polymorph screening
- Crystallization optimization
Wilmington PharmaTech has supported the registration of more than 30 patents on behalf of our customers. We have developed more than 300 API candidates, and supported upwards of 180 investigational new drugs (INDs), and 7 new drug applications (NDAs)

At the request of many clients, Wilmington PharmaTech has created a unique catalogue of difficult to synthesize starting materials and analogues available at Kg scale to facilitate discovery and IND enabling activities. A pdf list of these compounds is available upon request.
Wilmington PharmaTech by the numbers
0+ years
of cGMP manufacturing
0+ API
candidates developed
0+ patents
filed for clients
> 0
IND submissions, and counting…
About Us
Better Science, Better Results
With deep scientific experience developed across hundreds of molecules, Wilmington PharmaTech provides flexible sourcing and support options coupled with close attention to each client’s objectives and each molecule’s needs.
Our customers choose Wilmington PharmaTech because for over 20+ years, we have demonstrated we have the science and the capabilities to help make their projects real. Our customers value our flexibility in finding ways to accelerate their projects, and a proven quality management system (QMS) with a history of delivery excellence.
Our 54-acre, FDA-inspected, Delaware Campus features state-of-the-art cGMP-compliant API manufacturing capacity for clinical, specialty-scale, and commercial demand, plus dedicated facilities, including:
- Facilities dedicated to development- and clinical-scale manufacturing, with full analytical support
- A building dedicated to late stage/larger capacity clinical and commercial supply, with state-of-the-art HPAPI suites operating to occupational exposure band (OEB) 5 level.
A dedicated and experienced team…
We provide fast, local access to scientists with a wealth of expertise and a combined 20+ years of experience developing more than 300 API candidates, plus agile U.S.-based project management.
…and World-Class Facilities
Our deep heritage is there for all to see. We have invested in our already well-equipped ex-DuPont/Merck laboratories and cGMP suites to include continuous flow chemistry capabilities and a dedicated pilot plant.
We give you better science, faster.
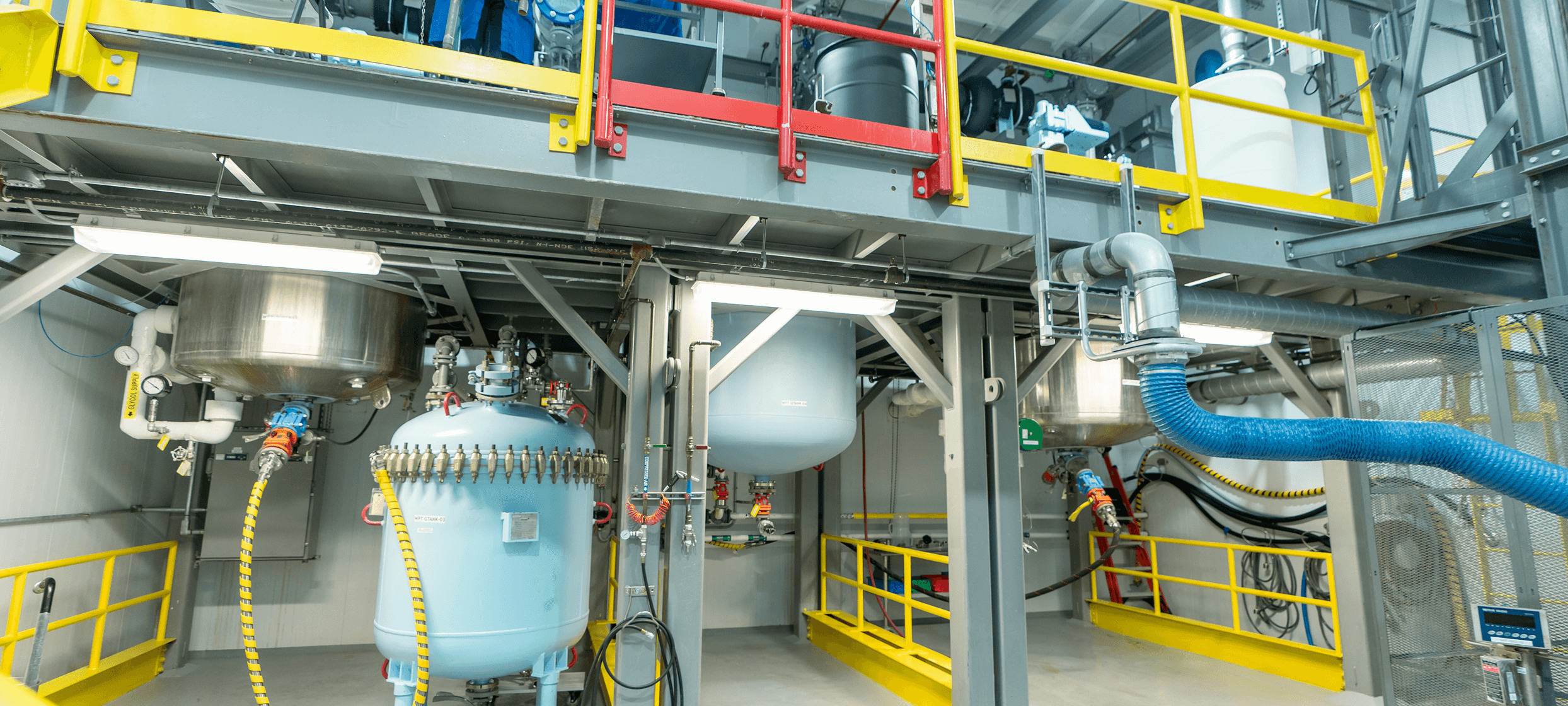

Pencader – US Development and Clinical Manufacturing Facility
DTP – US Commercial Manufacturing Facility

Suzhou – China Accelerated Development and Analytical Support Facility
Our business has been built upon trusted performance, creating long-term relationships with many large pharma companies, and dozens of smaller innovators.
Harry Li, Ph.D,
CEO & Founder of Wilmington Pharmatech Company

Our 20+ years of history
Company Started
DTP Solid State Lab Started
DTP Large Process Lab Started
DTP cGMP Kilo Lab Completed
Leased Pencader, DE Site
Pencader Process Lab Started
Pencader Kilo cGMP Lab Started
Pencader Facility Completed.
Headquarters Moved to Pencader Facility
China Suzhou Research Center
New Pilot Production Facilities in Zhangjiagang, Suzhou
New Glasgow facilities with both pilot plant and HPAPI suits in Newark, DE.
Careers
At Wilmington PharmaTech, we are constantly looking to hire talented and experienced synthetic and analytical chemists who have a passion for research and development.
Send an Inquiry
Could we be at your service?

